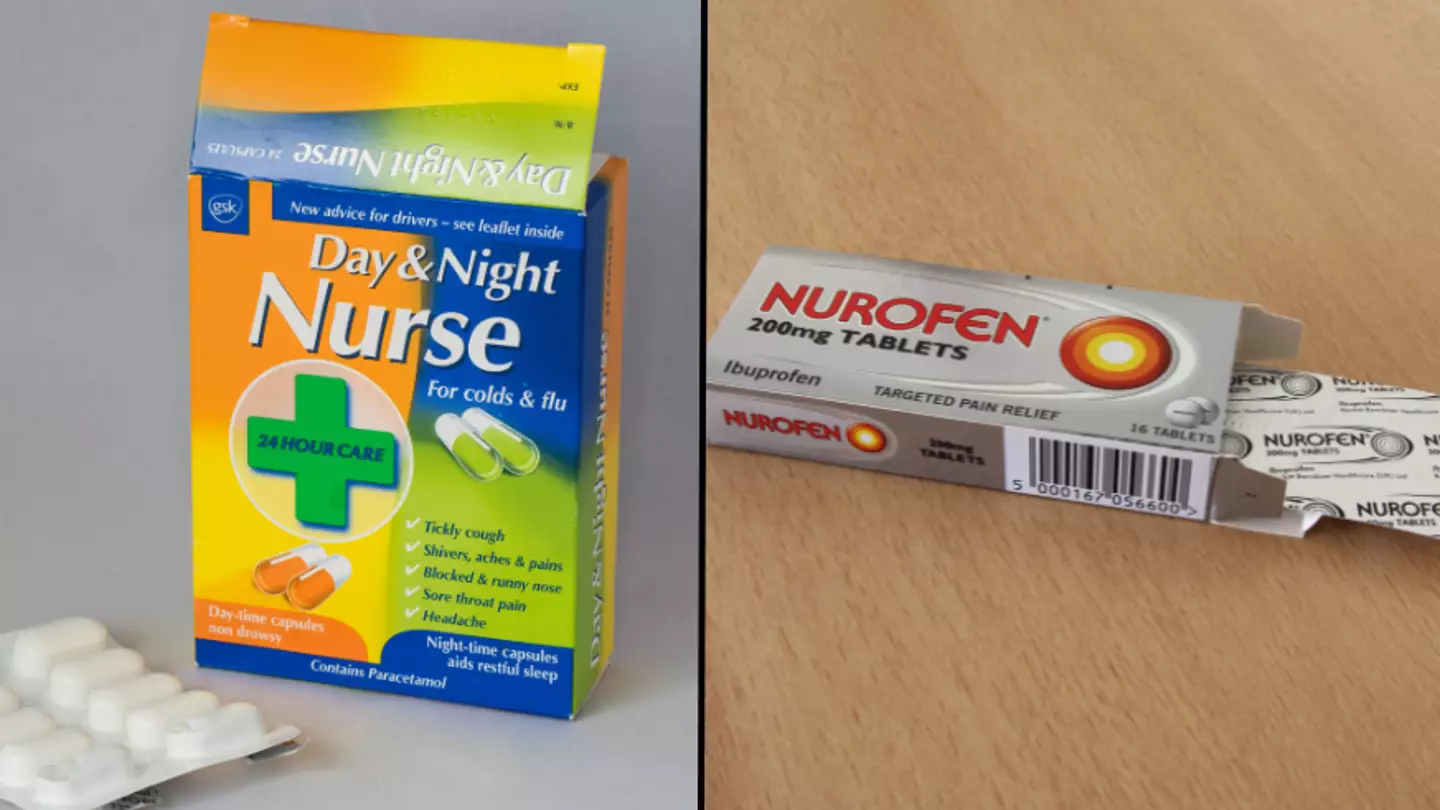
Over-the-counter medicines such as Sudafed and Night Nurse are under review by health watchdogs amid concerns about links to brain disorders.
An investigation was launched by the Medicines and Healthcare Products Regulatory Agency (MHRA) after the European Medicines Agency (EMA) announced earlier this month that it was looking in to medicines containing pseudoephedrine.
Customers are not being told to stop using the products while the review is ongoing.
Pseudoephedrine is the thing we so often rely on when struck down with a cold, when it feels like your head is stuffed full of cotton wool and only a decongestant will do the trick.
Advert

The medicine helps you breathe more easily if your nose is stuffy or blocked, which happens when blood vessels in your nose become swollen.
Pseudoephedrine works by reducing this swelling, with the drug found in a range of over-the-counter medicines including Sudafed, Night and Day Nurse and Nurofen Cold and Flu.
The EMA launched its review following concerns over pseudoephedrine's risk of posterior reversible encephalopathy syndrome (PRES) and reversible cerebral vasoconstriction syndrome (RCVS), conditions which affect blood vessels in the brain.
Both are extremely rare, but common symptoms associated with them include headache, nausea and seizures, and they can also cause major and life-threatening complications in some cases.
The MHRA had received two reports of the conditions linked to patients who had used pseudoephedrine products; one who had PRES and recovered, and the second who had RCVS, whose outcome was reported as unknown.
Customers who buy medicines containing pseudoephedrine are warned in the product information that they have a known risk of side effects involving ischaemia in the heart and brain, but the EMA's review aims to determine whether the medicines should be 'maintained, varied, suspended or withdrawn across the EU' as a result of the seriousness of PRES and RCVS.

The MHRA's review is based on the available evidence on potentially serious side effects, with a spokesperson for the agency assuring that it keeps 'the safety of all medicines under close review to ensure that the benefits outweigh any risks'.
"The safety of the public is our top priority," the spokesperson continued.
“We are reviewing the available evidence regarding the use of medicines containing pseudoephedrine and the risk of posterior reversible encephalopathy syndrome (PRES) and reversible cerebral vasoconstriction syndrome (RCVS), which have been very rarely reported with these medicines. We will provide any further advice as appropriate.
“We would also like to remind patients and parents/carers to report any suspected side effects to our Yellow Card scheme.”
LADbible has reached out to Night Nurse, Nurofen and Sudafed for comment.